Where are you?
To give you the most accurate information about our products and what we can do for you, we need to direct you to the right site.
To give you the most accurate information about our products and what we can do for you, we need to direct you to the right site.
Product information on this website is intended only for healthcare professionals and medical devices distributors. This website may contain general information relating to medical conditions and their treatment. Such information is provided for informational purposes only and is not meant to be a substitute for advice provided by a doctor or other qualified health care professional. If you are a patient or carer of a patient please consult your doctor for professional medical advice.

MagnetOs has achieved nearly twice the fusion rate of autograft in the latest level 1 clinical trial.1
1Data on file. Results are awaiting statistical analysis and peer-review

The future of spinal fusions is right here
Easing the burden of spine-related pain
Spine-related pain is taking a huge toll on our society: more bed days, more days off work, and at a greater financial cost to westernized healthcare than any other condition. In fact, today, nearly 1 in 5 spinal fusions fail.1,2
Here at Kuros Biosciences, we’re on a mission to discover, develop and deliver innovative biologic fusion technologies. And here’s how:
MagnetOs is a bone graft like no other: thanks to its NeedleGrip™ surface technology, it grows bone even in soft tissues – for a more predictable fusion.*†‡§3-5
A proven scientific
track record
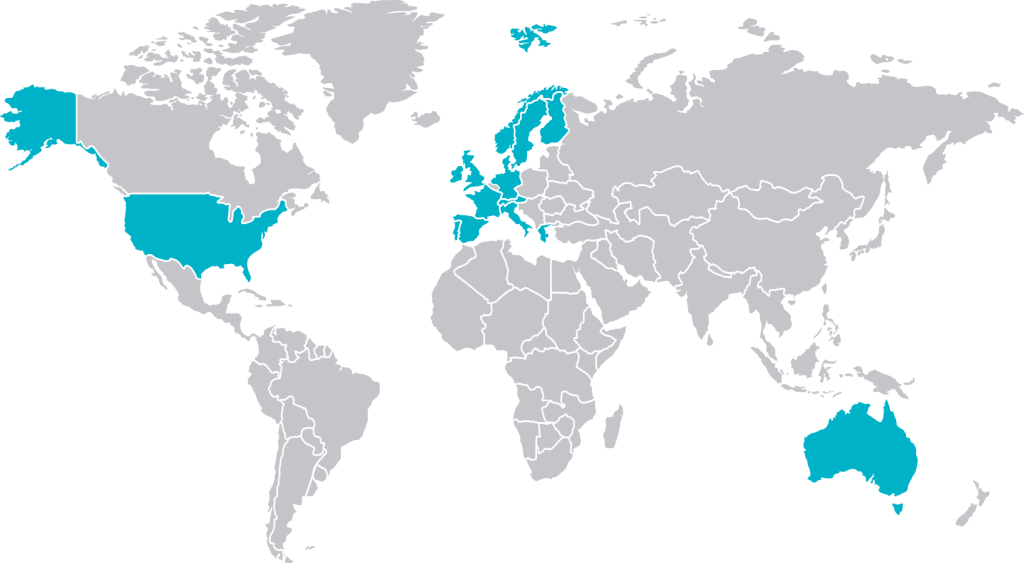
We are a Swiss-headquartered global innovation company with 150 years’ combined research experience in the field of biologics for spinal fusion. Our commercial & research footprint spans three continents and covers 19 markets.
Project Fusion: where Science & Clinical Evidence meet
We believe that a greater quality and quantity of science holds the key to easing the burden of spine surgery – especially as high non-union rates force surgeons, medical organizations and insurers to reassess the bone grafts they choose. Our response? Project Fusion: a global research program that brings together an unprecedented blend of scientific, pre-clinical and clinical studies.
Strategic Advisory Board
Our work is supported by some of the world’s pre-eminent spinal surgeons and experts.
Find out more
about Kuros
For further information, send us your details, and we will be in touch.
1. Medtech 360 report "Orthopedic Biomaterials Market Analysis 2017".
2. Hsu, et al. GSJ. 2012;2:239-248.
3. Van Dijk, et al. eCM. 2021;41:756-73
4. Duan, et al. eCM. 2019;37:60-73.
5. Van Dijk, et al. Clin Spine Surg. 2020;33(6):E276-E287.
*Results from in vitro or in vivo laboratory testing may not be predictive of clinical experience in humans. For important safety and intended use information please visit kurosbio.com/eifu.
†MagnetOs is not cleared by the FDA or TGA as an osteoinductive bone graft.
‡MagnetOs has been proven to generate more predictable fusions than two commercially available alternatives in an ovine model of posterolateral fusion.
§In large animal models.
PROMO/KUR/GL/062-21/R04